According to the Gay-Lussac which establishes that the pressure of a fixed volume of a gas is directly proportional to its temperature.
It is represented by the following formula:
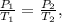
The pressure must be in atm units and temperature in kelvin. We calculate the conversion of each one:
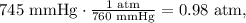
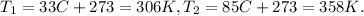
The final step is clear the final pressure which is P2 and replace the values there:
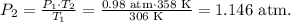
Then, we do the conversion from atm to mmHg:
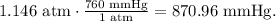
The final pressure is 870.96 mmHg.