Use the Planck expression to find the energy of a photon.
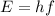
Where h = 6.63x10^-34 J*s and f = c/lambda.
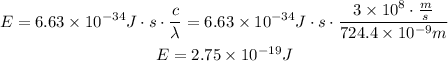
Now, transform the energy from Jules to electron-volts. We know that 1 electron-volt equals 1.60x10^-9 J.
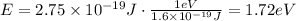
Therefore, the energy of the photon with the given wavelength is 1.72 eV.