Answer:
Isotonic solutions are the solutions which have same salt concentration or having same osmotic pressure across the semi-permeable membrane.
Now, as we know, the molarity of intracellualar fluids of potato is approximately equals to 0.2 M. Hence, the salt solution should have approximately 0.2 M concentration in order to achieve same osmotic pressure.
Molecular mass of salt (NaCl) = 58.44 g/mol
Molarity = 0.2 M
Volume of solution = 100 ml
Now, molarity =
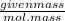 x
x
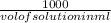
Hence, 0.2 = (given masss)/58.44 x (1000/100)
11.688/10 = given mass
Given mass = approx 1.17 gm
Hence, 1.17 % salt solution would most likely to be isotonic to the intracellular fluids of potato.