Answer:
The correct answer is oxidation-reduction.
Step-by-step explanation:
This reduction-oxidation reaction is given by the following semi-reactions:
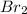 + 2
+ 2
 → 2
→ 2
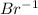 (reduction)
(reduction)
2
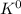 - 2
- 2
 → 2
→ 2
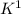 (oxidation)
(oxidation)
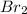 is an oxidation agent, K is a reducing agent.
is an oxidation agent, K is a reducing agent.
This means that
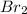 oxidizes K by increasing its oxidation number from 0 to 1.
oxidizes K by increasing its oxidation number from 0 to 1.
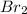 is reduced, decreasing its oxidation number from 0 to -1.
is reduced, decreasing its oxidation number from 0 to -1.
Have a nice day!