Answer : The mass of iron(III)sulfide is, 5.4288 g
Solution : Given,
Mass of iron, Fe = 3 g
Mass of sulfur,
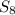 = 2.5 g
= 2.5 g
Molar mass of Fe = 56 g/mole
Molar mass of
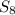 = 256 g/mole
= 256 g/mole
Molar mass of iron(III)sulfide,
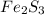 = 208 g/mole
= 208 g/mole
- The balanced chemical reaction is,
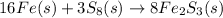
First we have to calculate the moles of iron and sulfur.
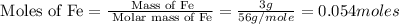
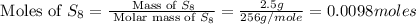
- From the balanced reaction, we conclude that
16 moles of Fe react with 3 moles of
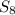
0.054 moles of Fe react with
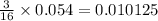 moles of
moles of
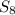
Therefore, the excess reagent in this reaction is, Fe and limiting reagent is,
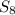
Now we have to calculate the moles of FeS.
As, 3 moles of
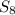 gives 8 moles of
gives 8 moles of
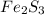
So, 0.0098 moles of
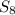 gives
gives
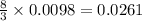 moles of
moles of
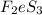
The moles of
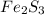 = 0.0261 moles
= 0.0261 moles
Now we have to calculate the mass of
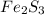 .
.
Mass of
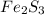 = Moles of
= Moles of
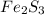 × Molar mass of
× Molar mass of
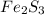
Mass of
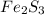 = 0.0261 g × 208 g/mole = 5.4288 g
= 0.0261 g × 208 g/mole = 5.4288 g
Therefore, the mass of iron(III)sulfide is, 5.4288 g