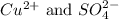Answer: The ions which are present in the solution of
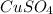 are
are
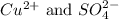 , both in aqueous state.
, both in aqueous state.
Step-by-step explanation: When
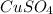 is dissolved in water, it forms an aqueous solution. The solution contains two ions, both in aqueous states.
is dissolved in water, it forms an aqueous solution. The solution contains two ions, both in aqueous states.
Equation follows:
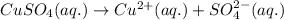
Ions that are present in the solution of
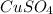 are
are