Answer:The correct answer is option A.
Step-by-step explanation
Mass of the iron pyrite= 20 grams
Volume water displaced by the iron pyrite = 4 mL
Density of iron pyrite=
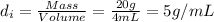
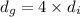 (Given)
(Given)
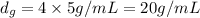
Mass of gold = 20 g
Volume of water displaced by the 20 grams of water = V
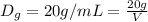
V = 1 mL
20 grams of gold will displace 1 ml of water.So, the correct answer is option A.