Answer:Total atoms in 131.97 liters of water vapor at STP are
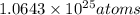 .
.
Step-by-step explanation:
At STP, one mole of gas occupies 22.4 L of volume.
Then 131.97 L of the volume will be occupied by:
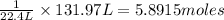
Number of water vapor molecules :
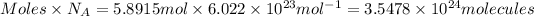
In 1 molecule of water vapor = 3 atoms
Total number of atoms in
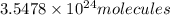 :
:
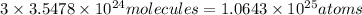
Total atoms in 131.97 liters of water vapor at STP are
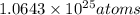 .
.