Answer : The correct option is, 55.92 %
Solution : Given,
Molar mass of
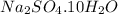 = 322 g/mole
= 322 g/mole
Molar mass of water = 18 g/mole
The mass of
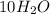 =
=
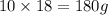
Now we have to calculate the percent by mass of water in
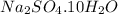
Formula used :
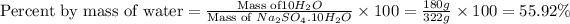
Therefore, the percent by mass of water in
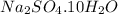 is, 55.92 %
is, 55.92 %