Given:
Initial temperature of ice,
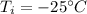
Mass of ice,
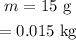
Final temperature,
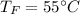
The heat absorbed to bring the temperature of ice to 0°C is given as,
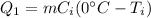
Here, C_i is the specific heat of ice.
Substituting all known values,
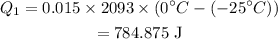
The heat absorbed to melt ice is given as,
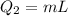
Here, L is latent heat of fusion of ice.
Substituting all known values,
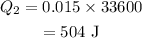
The heat absorbed by water to raise its temperature to 55°C is given as,
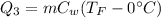
Here, C_w is the specific heat of water.
Substituting all known values,
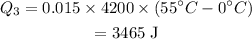
Therefore, the heat absorbed the ice to raise the temperature for -25° C to 55°C is ,
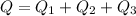
Substituting all known values,
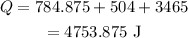
Therefore, the the heat absorbed the ice to raise the temperature for -25° C to 55°C is 4753.875 J.
Phase change diagram: