Answer: A.) 0.029
Explanation: To calculate the moles, we use the equation:
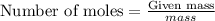
For sodium:
Mass of sucrose given = 10 g
Molar mass of sucrose
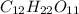 = 342 g/mol
= 342 g/mol
Putting values in above equation, we get:
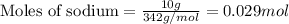
Thus the number of moles of sucrose available for this reaction will be 0.029.