Answer:
Option b

Step-by-step explanation:
Covalent compounds are formed by the sharing of electrons while ionic compounds are formed by transfer of electrons.
In the give compound,
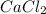 , two electrons are transferred from Ca to two Cl atoms. Hence , it is an ionic compound.
, two electrons are transferred from Ca to two Cl atoms. Hence , it is an ionic compound.
In the compound,
 , bond is formed by the sharing of electrons. Oxygen shares two of its electrons while two hydrogens share one electron each during the formation of covalent bond.
, bond is formed by the sharing of electrons. Oxygen shares two of its electrons while two hydrogens share one electron each during the formation of covalent bond.
In the compound NaOH, one electron is transferred from Na. Hence it is an ionic compound.
In the compound, LiF, one electron is transferred from Li to F. hence it is an ionic compound.
Among, the given options, only
 is a covalent compound.
is a covalent compound.