Answer:
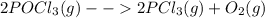
Step-by-step explanation:
Hello,
For the listed chemical reactions, one can predict the one which has a positive ΔS° is:
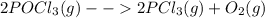
This is foreseen owing to the greater number of products (2) by contrast with the number of reagents (1). Moreover, for such reaction, there is a greater amount of moles at the products than at the reagents.
Best regards.