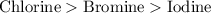Answer: The order of reactivity of non-metals from most reactive to least reactive is
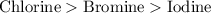
Step-by-step explanation:
Reactivity of a non-metal is defined as the tendency of an element to gain electrons. The reactivity increases as we move across a period and it decreases as we move down the group.
When the size of an element increases, the valence electrons gets away from the nucleus and the tendency of an element to gain electrons decreases.
In a group, the size of an element increases because there is an addition of new shell and electron is added in that shell.
The given elements belong to the same group which is Group 17.
Chlorine has the smallest size, then bromine and then iodine.
Hence, the order of reactivity of non-metals from most reactive to least reactive is