Answer:
The correct answer is:It contains little or no HA.
Step-by-step explanation:
When a strong acid is added to water it dissociates into its constituent ion .
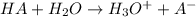
Actually the concentration of
 ions in water increases in comparison to hydroxide ions. And very little amount of HA will remain present associated in the solution.
ions in water increases in comparison to hydroxide ions. And very little amount of HA will remain present associated in the solution.