*Given
1 mole of HCl
*Solution
Chemical Reaction:
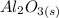
+
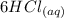
----->
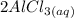
+
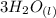
The chemical reaction is a double displacement reaction, where the oxide ions of the
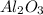
were "transferred" to the hydrogen ions of HCl to form
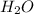
, while the chloride ions of HCl were "transferred" to the aluminum ions of
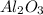
to form
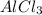
.
Arising from the law of mass conservation where the mass of the reactants must equal the mass of the products, chemical equations must therefore be balanced. Another way of interpreting the law of conservation of mass is that the number of moles for every ELEMENT (not molecule or compound) in the reactants, must equal the number of moles of every ELEMENT in the products. This principle allows us to balance chemical reactions.
Looking at the chemical reaction above, we can see that the 2 moles of Al in the reactant is balanced by 2 moles of Al in the product; 3 moles of O in the reactant is balanced by 3 moles of O in the products; and finally, 6 moles of H, and 6 moles of Cl in the reactant, are also balanced by 6 moles of H, and 6 moles of Cl in the products, respectively.
For every mole of HCl,
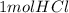
X
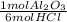
= 1/6
Thus, 1 mol of HCl would need 1/6 mol of
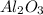 to completely react and form products.
to completely react and form products.