Answer:
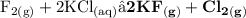
Step-by-step explanation:
» The prediction is 98% correct because single displacement reaction type is highly possible.
This is because Fluorine has is more electronegative than Chlorine in Potassium Chloride. So, it strongly displaces Chlorine from the solution hence forming Chlorine gas.
» The 2% of wrong prediction maybe because of wrong reactant measurements following mole concept chemistry.
If you are asked the observation,
Observation » A green yellowish gas is formed.
This gas is Chlorine gas (Cl2)