Answer: Option (a) is the correct answer.
Step-by-step explanation:
When a neutral atom loses an electron then it acquires a positive charge.
For example, sodium on losing an electron become
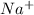 ion.
ion.
On the other hand, if a neutral atom gains an electron then it acquires a negative charge.
For example, a neutral chlorine atom on gaining an electron forms
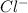 .
.
Hence, an ion is formed in both the cases.
Thus, we can conclude that in the given situation these charged atoms called ions.