Answer:
13.09 moles of HNO3 are produced.
Explanation:
1st) It is necessary to balance the chemical equation:
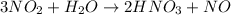
Now we know that 3 moles of NO2 react with 1 mole of H2O to produce 2 moles of HNO3 and 1 mole of NO.
2nd) The percent yield rate, it is used to demonstrate that the reaction is not 100% efficient. According to the formula of percent yield rate, we have that:
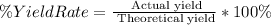
So, we know that the percent yield rate for this reaction is 96%.
3rd) With the stoichiometry of the balanced equation, we can calculate the Theoretical yield:
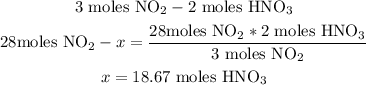
Now we know that with 28 moles of NO2, 18.67 moles of HNO3 are produced if the reaction was 100% efficient.
With the molar mass of NO2 (46g/mol) we can convert moles to grams:
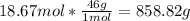
4th) With the 858.82 grams of NO2 and the percent yield rate, we can calculate the Actual yield of this reaction:

5th) Now that we know the actual yield of the reaction, we can calculate the moles of HNO3 that are produced, using the molar mass of HNO3 (63.01g/mol):
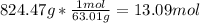
So, 13.09 moles of HNO3 are produced.