Step-by-step explanation:
Atomic number of calcium is 20 and its electronic distribution is 2, 8, 8, 2. Hence, to attain stability it will readily lose its valence electrons.
Hence, neutral Ca atom will change into
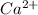 ion.
ion.
Whereas atomic number of oxygen is 8 and its electronic distribution is 2, 6. Hence, to attain stability it will needs to gain two electrons.
Hence, neutral O atom will change into
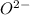 ion.
ion.
Therefore, when both calcium and oxygen atoms will chemically combine with each other then according to cross multiplication same charges will be cancelled out resulting in the formation of calcium oxide (CaO) compound.
Thus, we can conclude that charge on calcium in this compound is +2.