Answer : The correct answer will be A =3 and B =2 as the electron configuration of Mg will be as
![[Ne] 3s^(2)](https://img.qammunity.org/2018/formulas/chemistry/college/2uth8wz5s8768fauy7l50xamlbkmsfm2i1.png) where A is 3 and B is 2.
where A is 3 and B is 2.
Explanation :
Magnesium has atomic number as 12. The complete electron configuration will be as
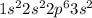 .
.
which can be abbreviated as
![[Ne] 3s^(2)](https://img.qammunity.org/2018/formulas/chemistry/college/2uth8wz5s8768fauy7l50xamlbkmsfm2i1.png) as the electron configuration resembles to that of neon element.
as the electron configuration resembles to that of neon element.
In the outermost shell the electron in 3 s orbital is only 2. So, therefore 3 is the orbital of S and there are 2 electrons in it.