Answer: The correct answer is Option 3 and Option 4.
Step-by-step explanation:
Redox reactions are termed as the reactions in which reduction and oxidation reactions occur simultaneously.
Reduction reactions are the ones where a substance gains electrons. The oxidation state of the substance gets reduced.
Oxidation reactions are the ones where a substance looses electrons. The oxidation state of the substance is increased.
Fro the given options:
Option 1:
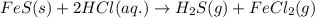
This reaction is a type of double displacement reaction because exchange of ions takes place.
Option 2:
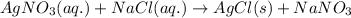
This reaction is a type of double displacement reaction because exchange of ions takes place.
Option 3:
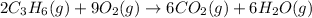
On reactant side:
Oxidation state of Carbon = +2
Oxidation state of Oxygen = 0
On product side:
Oxidation state of Carbon = +4
Oxidation state of Oxygen = -2
Here, oxidation state of carbon is increasing from +2 to +4. Thus, it is getting oxidized and oxidation state of oxygen is getting reduced from 0 to -2. Thus, it is getting reduced.
Option 4:
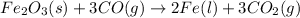
On reactant side:
Oxidation state of Iron = +3
Oxidation state of Carbon = +2
On product side:
Oxidation state of Iron = 0
Oxidation state of Carbon = +4
Here, oxidation state of carbon is increasing from +2 to +4. Thus, it is getting oxidized and oxidation state of iron is getting reduced from +3 to 0. Thus, it is getting reduced.
Hence, the correct answer is Option 3 and Option 4.