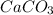Answer:
D.
Step-by-step explanation:
We are given that a chemical reaction
_
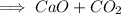
We have to find the value of reactant for given product.
Reactant: The substance on the left side in chemical reaction are called reactants.
Product: The substance on the right side in chemical reaction are called products.
In given product, there are calcium oxide and carbon dioxide.
Therefore, calcium, oxygen and carbon must be present in reactants.
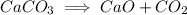
Hence, option D is true.
Answer: D.