Answer:
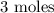
Step-by-step explanation:
Here, we want to know the number of moles of nitrogen needed to produce 9 moles of ammonia
From the equation of reaction given:
1 mole of nitrogen gas produced 3 moles of ammonia gas
Thus:
x moles of nitrogen gas will produce 9 moles of ammonia gas
Mathematically:
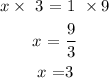
What this means is that 3 moles of nitrogen gas are required to produce 9 moles of ammonia gas according to the given equation