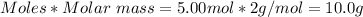Answer:
Theoretical yield of H2 = 10.0 g
Step-by-step explanation:
Given:
Moles of Zn added = 5.00
To determine:
The theoretical yield of H2 gas
Calculation:
The balanced reaction between Zn and HCl is as shown below:
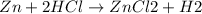
Based on the reaction stoichiometry:
Since HCl is in excess, the limiting reagent is Zn. Thus,
1 mole of Zn will produce 1 mole of H2
Accordingly, 5 moles of Zn will produce 5 moles of H2
Molar mass of H2 = 2 g/mol
Mass of H2 produced =