Answer:
-0.1051
Step-by-step explanation:
First, we need to find the degree of dissociation (α) of each of the groups using the Handerson-Halsebach equation:
pH = pKa + log (α/1-α)
log (α/1-α) = pH - pKa
log (1-α/α) = pKa - pH
1-α/α =
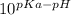
1-α =
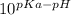 *α
*α
α =
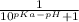
So, fo the α-carboxyl group:
α =
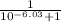
α = 1.0000
And for the α-amino group
α =
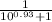
α = 0.1051
In the ionization, the α-carboxyl group goes from neutral to a negative ion with charge -1, and the α-amino group goes from a positive ion with charge +1 to a neutral compound. So, the net charge is how many ions are presented multiplied by the charge.
For the first one, the value of α is the value of ions, but for the second, α represents the neutral, so the ions are 1-α:
-1*(1.000) + (+1)*(1-0.1051) = -0.1051