Answer : The volume of water added = 9.411 ml
Solution : Given,
Absorbance decreases by 32% upon dilution means,
Let, initial absorbance = 100
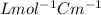
then final absorbance = 100 - 32 = 68
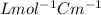
initial concentration = 1.2 M
initial volume = 20 ml = 0.02 L ( 1 L = 1000 ml )
According to Beer-Lambert law, the absorbance is directly proportional to the concentration of an absorbing species.
A ∝ C
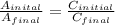
Now put all the given values in this formula, we get
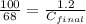
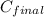 = 0.816 M
= 0.816 M
Now, calculating the number of moles,
Moles = concentration × volume
Moles = 1.2 × 0.02 = 0.024 moles
Now, Calculating the final volume by this formula.
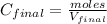
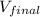 =
=
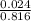 = 0.0294 L = 29.411 ml
= 0.0294 L = 29.411 ml
The inital volume is 20 ml and final volume is 29.411 ml.
Volume of water added = final volume - initial volume = 29.411 - 20 = 9.411 ml