Answer: This is known as non-polar covalent bond.
Step-by-step explanation:
A covalent bond is formed when sharing of electrons takes place between the atoms forming a bond.
A polar covalent bond is formed when the atoms forming a bond have significant difference in electronegativities. This leads to the formation of poles in the atoms. For Example: HCl
A non-polar covalent bond is formed when the atoms forming a bond dpes not have significant difference in electronegativities or have similar electronegativity. For Example:
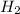
Hence, the bond is known as non-polar covalent bond.