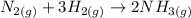
By using the ideal gas law to get volume we have"
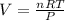
Where v is volume, T is temperatute, n is number of moles, R is the molar gas constant and P is pressure. At STP P= 101,325 Pa, T= 273.15 K and R= 8.314 J/mol K
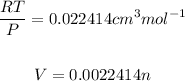
We must first convert mass to moles:
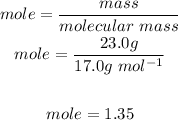
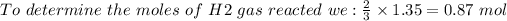
By substituting this value into the ideal gas law we have:
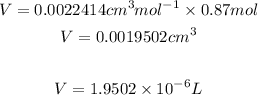
1.9502e-6L of H2 gas reacted at STP