Answer : The correct option is, (d) He and Be
Explanation :
First we have to determine the electronic configuration of the following elements.
The electronic configuration of hydrogen (H) is:
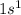
The electronic configuration of carbon (C) is:
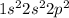
The electronic configuration of lithium (Li) is:
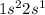
The electronic configuration of helium (He) is:
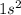
The electronic configuration of beryllium (Be) is:
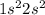
From the electronic configuration of the elements we conclude that, the hydrogen element has '1' valence electron, carbon element has '4' valence electrons, lithium element has '1' valence electron, helium element has '2' valence electrons and beryllium element has '2' valence electrons.
Thus, the helium and beryllium are the elements that holds up to two electrons in their outermost shell.
Hence, the correct option is, (d) He and Be