Answer: The given statement is false.
Step-by-step explanation:
When an element chemically combines with another element then it results into the formation of a molecule or a compound.
A compound is defined as the substance in which different elements are chemically combined together in a fixed ratio by mass.
For example,
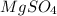 is a compound and elements are present in 1:4 ratio.
is a compound and elements are present in 1:4 ratio.
A compound can be divided into its constituent or simpler substances by chemical means.
And, a molecule is defined as the substance in which two or more same type of elements chemically combine together in a fixed ratio by mass.
For example,
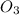 ,
,
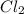 etc are molecules.
etc are molecules.
Therefore, we can conclude that the statement two or more atoms bonded together always form a compound, is false.