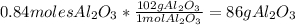Answer:
86g of
Step-by-step explanation:
1. The balanced chemical equation is:
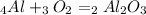
2. Use the stoichiometry of the reaction to calculate how many moles of aluminum oxide can be formed.
If the oxygen is in excess it means that the aluminum is the limiting reagent and the calculations must be made using the mass of aluminum.
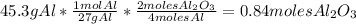
3. Use the molar mass of the
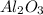 to calculate how many grams of aluminum oxide can be formed:
to calculate how many grams of aluminum oxide can be formed: