Answer:
Lowering the volume of H2 gas
Step-by-step explanation:
According to Le Chatelier's principle when a reaction is at equilibrium is subject to changes in temperature, pressure and concentration the equilibrium will then shift in a direction to undo the effect of the induced change.
The given reaction is:
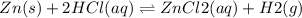
In order to decrease the rate of production of ZnCl2 the reaction needs to shift to the left. This can be achieved by lowering the volume of H2 gas which would increase its pressure. Based on Le Chatelier's principle, this would shift the equilibrium in a direction that would lower the H2 pressure i.e. to the left thereby also decreasing the production of ZnCl2.