Answer : The number of molecules of ammonia formed would be, 2.
Explanation :
As per question, when the hydrogen molecule react with nitrogen molecule then it gives ammonia as a product.
The balanced chemical reaction will be :
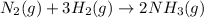
By the stoichiometry we can say that, 1 mole of nitrogen gas react with 3 mole of hydrogen gas to give 2 mole of ammonia gas as product.
The number of molecules of nitrogen gas = 1
The number of molecules of hydrogen gas = 3
The number of molecules of ammonia gas = 2
Therefore, the number of molecules of ammonia formed would be, 2.