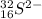Answer:
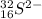
Step-by-step explanation:
Atomic number : It is defined as the number of electrons or number of protons present in a neutral atom.
However, when we talk about the atomic number of the ion, it is not equal to the number of electrons as electron can be gained or loosed.
This is why, more appropriately, the number of the protons which are present in the nucleus of the atom is called the atomic number.
Thus, number of protons = atomic number = 16
The element must be sulfur.
Since, number of protons is not equal to the number of electrons, thus the isotope will not be neutral.
There are 2 more electrons than the number of protons and thus, the isotope will be having a charge of -2.
Mass number is the number of the entities present in the nucleus which is the equal to the sum of the number of protons and electrons.
Mass number = Number of protons + Number of neutrons = 16 + 16 = 32 neutrons
The identity is:-