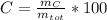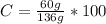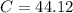Answer:
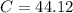 % of C
% of C
Step-by-step explanation:
Hi, the empirical formula of the pentaerythritol is {tex]C_5H_{12}O_4[/tex]
The molecular weights are:

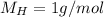
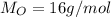
Due to the empirical formula in 1 mol of pentaerythritol you have 5 mol of C, 12 mol of H and 4 mol O
Taking a calculation base of 1 mol:

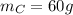
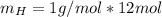
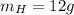
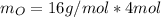
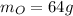
The total weight will be:
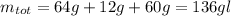
The C%: