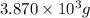Answer :
(1) The given number in 4 significant figures will be,
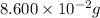
(2) The given number in 4 significant figures will be,

(3) The given number in 4 significant figures will be,
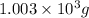
(4) The given number in 4 significant figures will be,
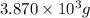
Explanation :
Significant figures : The figures in a number which express the value -the magnitude of a quantity to a specific degree of accuracy is known as significant digits.
(1) The given number 0.086 g converted into 4 significant figures.
In 0.086 g, there are 2 significant figures. Now we have to convert it into 4 significant figures.
The given number in 4 significant figures will be,
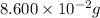
(2) The given number 0.431 g converted into 4 significant figures.
In 0.431 g, there are 3 significant figures. Now we have to convert it into 4 significant figures.
The given number in 4 significant figures will be,

(3) The given number 1003 g converted into 4 significant figures.
Now we have to convert it into 4 significant figures.
The given number in 4 significant figures will be,
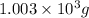
(4) The given number 3870 g converted into 4 significant figures.
Now we have to convert it into 4 significant figures.
The given number in 4 significant figures will be,