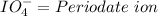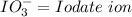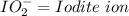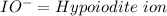Answer:
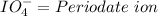
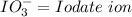
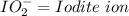
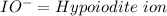
Step-by-step explanation:
Oxyanions:
Oxyanions are anions that have one or more oxygen atoms bonded to another elements is called oxyanions.
General formula of oxyanions are :
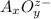
Where, A represents an element and O represents oxygen atom.
Four most common oxyanions of Iodine are: