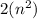Answer:
Eight electrons.
Step-by-step explanation:
An atom is composed by shells, each shell can contain a limited number of electrons. The first one can contain two electrons. The second shell contains up to eight electrons. The third shell contains up to 18.
There's a formula to calculate the number of electrons: