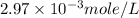Answer : The molarity of vitamin c in organic juice is,
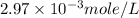
Solution : Given,
Mass of ascorbic acid (solute) = 124 mg = 0.124 g
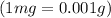
Volume of juice = 236.6 ml
Molar mass of ascorbic acid = 176.12 g/mole
Formula used :
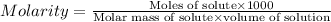
Now put all the given values in this formula, we get the molarity of vitamin c in organic juice.
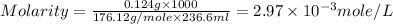
Therefore, the molarity of vitamin c in organic juice is,