The heat of reaction (∆H) is the energy that is released or absorbed when chemicals are transformed in a chemical reaction. In this case, we are told that heat is released from the vessel to the surroundings, i.e. we have an exothermic reaction. The heat of a reaction ∆H is equivalent to the heat measured by a heat meter. In this example, we are given this measured value, and assuming that at the beginning, before the reaction the heat released is zero and that there is no heat accumulation inside the vessel we can say that the ∆H of reaction will be equivalent to this value, 35kJ.
In the case of internal energy ∆E, we are told that the reaction is carried out at a constant volume, in this case, we can apply the next equation:
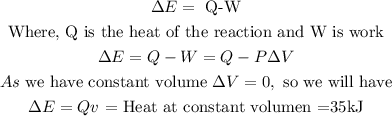
Therefore the value of ∆E will also be equivalent to 35kJ
Another important fact that we must take into account is that we are told that the reaction takes place in the gas phase, i.e. there is no phase change and therefore the heat of phase change will be 0