Given:
Half-life = 1620 years
Original mass, m₀ = 220 g
The decay equation is of the form
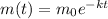
where
t = time, years
k = constant
At half-life, m = 0.5m₀ and t = 1620. Therefore

When t = 10 half-lives = 16200 years, obtain
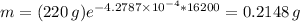
Answer: 0.215 g (nearest thousandth)