Answer:
Kr < Ar < Ne < N (N is fastest and Kr is slowest)
Step-by-step explanation:
According to graham law of diffusion, rate of diffusion of a gas is inversely proportional to the square root of molar mass of the gas.
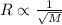
Where R is rate of diffusion and M is molar mass
Molar masses of given gases are - Ar(40 g/mol), Ne(20 g/mol), N(14 g/mol) and Kr(84 g/mol)
So molar mass order : Kr > Ar > Ne > N
Hence rate of diffusion order: Kr < Ar < Ne < N (N is fastest and Kr is slowest)