Step-by-step explanation:
1. Ca → element
Ca is a symbol of calcium element categorize as alkali earth metal.
2.
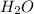 → compound
→ compound
Compound is a substance made up of two more than two different elements.
3. Nuclear decay → fission
Fission is defined as process in which heavier unstable nucleus divide itself into two or more smaller stable nuclei with release of large amount of energy.
4. Nuclear synthesis → fusion
Fusion is defined as process in which two or more nuclei combines together to form an larger nucleus with release of large amount energy.
5. η → neutron
One of the subatomic particle of an atom located in the nucleus of an atom with no electrical charge.
6. Positive charge → proton
One of the subatomic particle of an atom located in the nucleus of an atom with positive electrical charge.
7. e → electron
One of the subatomic particle of an atom located inside an atom and outside the nucleus of an atom with negative electrical charge.
8. Number of protons in nucleus → atomic number
Atomic number of an atom is equal to the total number of protons present in the nucleus if the an atom.