Answer Calcium chloride has the molecular formula
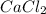 from which we can say that one molecule of calcium chloride consist of one atom of calcium and two atoms of chlorine.
from which we can say that one molecule of calcium chloride consist of one atom of calcium and two atoms of chlorine.
given : 2 mol of
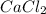
total moles of chlorine atoms = number of atoms in one mole × number of formula units= 2×2= 4
Total number of atom = Total number of moles × Avogadro number
So, total number of chlorine atoms =
4 × 6.0221×
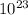 =2.4088×
=2.4088×
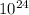 atoms
atoms