Answer:
The charge will be +2.
Step-by-step explanation:
The atomic number of Mg is 12, so it has 12 protons.
The neutral atom of Mg has 12 electrons.
When an atom loses electrons it acquire positive charge.
The charge developed is equal to the number of electrons removed.
So the charge developed will be +2.
The equation will be:
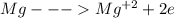
A negative charge is developed when atom accepts electrons.