Answer : The number of moles of phosphorous are 194 moles.
Explanation : Given,
Moles of
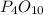 = 48.5 moles
= 48.5 moles
By the stoichiometry in
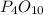 , there are 4 moles of phosphorus and 10 moles of oxygen.
, there are 4 moles of phosphorus and 10 moles of oxygen.
As, 1 mole of
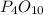 contains 4 moles of phosphorous
contains 4 moles of phosphorous
So, 48.5 moles of
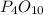 contains
contains
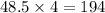 moles of phosphorous
moles of phosphorous
Therefore, the number of moles of phosphorous are 194 moles.