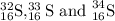Answer: The three isotopes of sulfur are
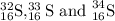
Step-by-step explanation:
Isotopes are defined as the substances which have same number of protons ( or Atomic number) but different number of neutrons (or Atomic mass). It is written in the form of
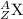
where,
Z is the atomic number
A is the atomic mass
X is the symbol of the element
Sulfur is the 16th element in the periodic table with atomic number 16.
For isotope 1: The atomic mass is 32
For isotope 2: The atomic mass is 33
For isotope 3: The atomic mass is 34
Therefore, the three isotopes of sulfur are