Step-by-step explanation
(a) The balanced equation of the reaction is:
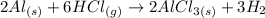
The compound F is solid AlCl₃
(b) When the solid AlCl₃ is heated to 900 °C, it changes from a solid to a gaseous state. The illustration of the change is shown below:
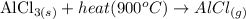
(c) Melting point of AlF₃ is high as compared to AlCl₃ because AlF₃ is an ionic compound while AlCl₃ is a covalent compound but the bond in AlCl₃ is a polar covalent bond. Melting points of ionic compounds are usually high as compared to covalent bonds because an ionic bond is stronger as compared to covalent bonds. Also, AlCl₃ has a simple molecular structure with weak van der Waals forces between its molecules. More energy is needed to overcome the strong ionic bonds in AlF₃ than the weak intermolecular forces in AlCl₃, hence AlF₃ has a much higher melting point.