Step-by-step explanation:
According to the mole concept, there are
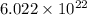 atoms present.
atoms present.
It is given that mass is 0.250 g. And, number of moles are equal to mass divided by molar mass.
Mathematically, No. of moles =
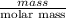
As molar mass of
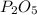 is 283.88 g/mol. Therefore, putting given values into the above formula as follows.
is 283.88 g/mol. Therefore, putting given values into the above formula as follows.
No. of moles =
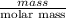
=
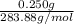
= 0.0008 mol
Hence, number of atoms present in 0.0008 mol are as follows.

= 0.0048 atoms
Thus, we can conclude that there are 0.0048 atoms in 0.250 g of
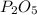 .
.